Enhanced Cooling Tower Maintenance Saves Water
Kathy | October 1, 2008
 Any amount of wasted water is too much. Perhaps it’s time to compare your maintenance procedures to current best practices. Do they measure up?
Any amount of wasted water is too much. Perhaps it’s time to compare your maintenance procedures to current best practices. Do they measure up?
Water is an undervalued utility. Since many consumers essentially view it as a “free” resource, many plants make little effort to conserve water the same way they try to conserve fuels and electricity. While countless opportunities to conserve Btus and kilowatts have been explored over the past 30 years, less attention has been paid to reducing water consumption. But things are changing…
Severe droughts, like those seen in the Southeast and Southwest, combined with rapid population and industrial growth in those and other water-stressed regions, have created a water crisis in many parts of the United States. The available supply of fresh water is decreasing as residential customers compete with industry for available water supplies. (Witness the lawn sprinkling and car washing bans in many communities. Agriculture, the largest consumer of water, increasingly relies on irrigation to offset drought conditions that limit yields. Residential customers accuse farmers of drawing down the water table and causing local shortages. Meanwhile, government regulatory agencies are hesitant to wade in to restrict water withdrawal rates or raise usage fees force conservation, since most consumers view the plentiful supply of safe, clean water as an inalienable right.
The conflict is intensifying. What’s worse, scientific and government forecasters predict no relief is in sight over the next decade. Blame it on global warming or not, the need to conserve water is here to stay.
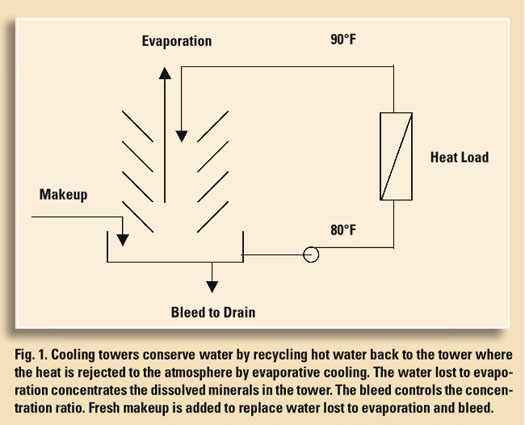
On the bright side, most plants already have a water conservation program under way. It’s called a cooling tower. As shown in Fig. 1, cooling towers conserve water by recycling it through equipment such as mechanical chillers, turbine condensers, air compressors, oil coolers and process heat exchangers. In the process, unwanted heat is removed and then rejected to the atmosphere by evaporative and convective cooling.
It is estimated that over 1,000,000 cooling towers—that’s one million—are in service. They are used in every conceivable application where water conservation is a concern, including electric utilities, oil refineries, steel mills, manufacturing facilities, pharmaceutical plants, food processing operations, hospitals, universities, commercial buildings and many more.
Interestingly, plant maintenance teams play a critical role in keeping their cooling towers operating at peak efficiency. They focus on keeping plant equipment clean by preventing mineral scale deposits, mitigating corrosion, limiting bacteria growth, and controlling fouling. Because of the growing importance of water conservation, however, best maintenance practices have been updated to cover the operation of cooling towers in a way that reduces fresh water withdrawal rates and produces less wastewater. That means if you are still maintaining your cooling tower the same way you did five years ago, it’s time to upgrade to the newer water conservation format.
Cooling tower basics Cooling towers work by evaporative and convective heat transfer. As water flows over the tower, some of it (about 0.1% of the flow) is evaporated to the atmosphere. About 1000 Btus of heat is removed for every one pound of water evaporated. Removing this heat from the bulk of the cooling water decreases the temperature by 10 to 15 degrees. On average, 75% of the heat rejected at the tower is by the evaporative cooling process; 25% of the cooling occurs due to the direct contact of cooler air with the warmer water. This varies, of course, based on weather conditions. If the air temperature is warmer than the water temperature, for example, all of the cooling takes place by the evaporative process. When the air is colder, such as during the winter, more heat is removed by convective heat transfer.
The water that is evaporated from the tower is pure—that is, it doesn’t contain any of the dissolved minerals present in the raw water supply. As the evaporation process continues, these minerals concentrate in the cooling water. If this is allowed to continue without limit, the dissolved minerals soon concentrate to a point where they can no longer remain in solution. Here the less-soluble minerals such as calcium and magnesium precipitate to form an insoluble sludge, or an adherent, dense scale in the tower basin or within plant equipment. For these reasons, cooling towers must be operated to keep the dissolved minerals soluble. This is accomplished by bleeding a small amount of tower water to drain while replacing the water lost by evaporation and bleed with fresh makeup. The ratio between the dissolved solids in the cooling water and the dissolved solids in the makeup is called the “concentration ratio,” “cycles of concentration” or, more simply, “cycles.” This is an indication of how efficiently the cooling tower is recycling water. A simple way to measure cycles is by calculating the ratio of the specific conductance of the cooling water to the specifi c conductance of the makeup. Alternatively, if the cooling tower has a water meter on the makeup and bleed lines, you can estimate cycles by computing the ratio between the gallons makeup to the gallons bleed. The cycles determined by either method should be in fairly good agreement assuming there are no uncontrolled water losses due to leaks or an overflow condition.
Establishing the proper operating limit for cycles is one of the supercritical decisions one faces in cooling tower maintenance. Towers that operate at high cycles use less water and produce less waste than towers that operate at lower cycles. The maximum permissible cycles of concentration are limited by the makeup water quality, bleed rate and uncontrolled water losses. Set the cycles too high and you risk running the tower under scale-forming conditions. Set it too low and you waste water, chemicals and energy.
Makeup water quality is key The quality of the cooling tower makeup determines the maximum cycles of concentration. Since calcium and magnesium are the primary scale-forming impurities, tower operation is confined to keeping the cycles below the solubility limit of calcium carbonate. This requires a proper balance to be maintained between the calcium hardness, total alkalinity, total dissolved solids and pH in the recirculated cooling water. Since these variables differ from one water supply to another, the limitation on cycles also varies. As a general rule of thumb, the calcium hardness is limited to 350 to 450 ppm in most cooling towers. Other limiting factors—such as silica, phosphate and process contaminates—need to be considered as well.
Generally, cooling towers use potable water as their makeup supply. This is a good choice when a plentiful supply of fresh water is available. But, as competition for clean, fresh water heats up, plants are increasingly forced to use alternative sources. Reclaimed water from municipal or industrial wastewater treatment plants is often an alternative source for cooling tower makeup. Although this reduces fresh water withdrawal rates, some pre-treatment of the wastewater may be required to make it suitable for use in the cooling tower. In many cases, regeneration by simple filtration may be all that is necessary to reuse wastewater as cooling tower makeup. Reverse osmosis (RO) continues to gain favor as a method for pre-treating makeup for steam boilers. The RO process produces a continuous supply of softened and dealkalized water for this application. The downside of RO is that it produces a continuous waste stream that is sent to drain—25% of the feedwater to the RO is lost to drain, while 75% is recovered for use as boiler makeup. In many cases, the RO reject is perfectly acceptable for use as cooling tower makeup. Although dissolved solids in the RO reject are about four times that of the RO feedwater, if the water is softened ahead of the RO to remove calcium and magnesium, it is of acceptable quality for reuse in this manner.
If the plant does not produce enough recycled wastewater to meet the cooling tower demand, the wastewater may be combined with fresh water to produce a suitable blend. Using an alternative water supply in this way reduces fresh water consumption and wastewater generation.
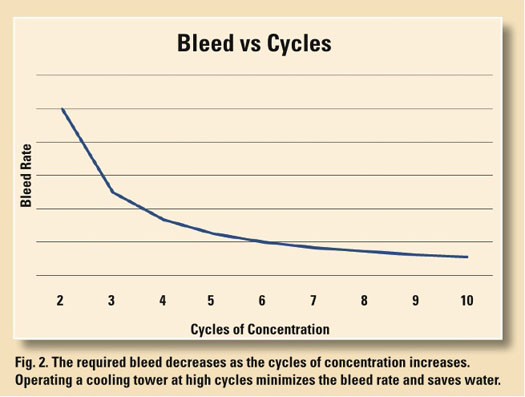
Maximize cycles to reduce water consumption Obtaining maximum performance from a cooling tower requires it to be operated at the maximum permissible cycles of concentration. Since the concentration ratio is determined by the ratio between makeup and bleed (C=MU/B), reducing the bleed increases the cycles, and conversely, increasing the bleed decreases the cycles. As shown in the Fig. 2 of bleed versus cycles, a point of diminishing returns is soon reached at about 10 cycles of concentration. From a practical view, it is difficult to operate a tower at more than 10 cycles because of leaks, windage and other uncontrolled water losses that contribute to the “bleed” and thereby limit cycles. If 10 cycles of concentration is taken as our target, then cooling towers that operate at less than 10 cycles can be considered as less than 100% efficient as determined by freshwater consumption and wastewater generation.
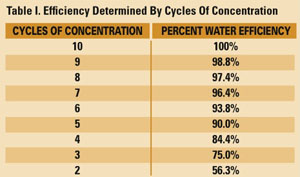
Table I reflects measures of tower efficiency as determined by the cycles of concentration, if we assume 10 cycles represents a practical 100% water efficiency rating. This table suggests that cooling towers that use freshwater makeup and operate at less than four cycles are not achieving their full potential. This condition wastes water, consumes excess chemicals and produces more wastewater. Towers that operate at five to eight cycles are acceptable for most applications, although large systems can save significant volumes of freshwater by reaching eight cycles. Towers that are maintained at nine to 10 cycles are operating at peak efficiency in terms of water consumption, chemical usage and energy demand.
Strategies to achieve cycles goal If the cooling tower is on the low end of the efficiency scale, several maintenance strategies are available to increase cycles, including pH adjustment and softening the makeup.
The standard method for increasing cycles on high hardness, high-alkalinity makeup is to use a strong mineral acid—such as sulfuric or hydrochloric—to reduce alkalinity and control pH. Calcium carbonate scale forms as a result of the chemical reaction between calcium hardness and carbonate alkalinity. By neutralizing the alkalinity with acid, the chemical reaction leading to calcium carbonate is stopped. Enough acid is injected into the tower makeup to reduce the total alkalinity to 50 to 100 ppm and maintain the pH of the cooling water within the range of 6.8 to 7.5.
Acid also has the reputation for being “forgiving” in that if scale deposits should form, a slight drop in pH will help remove them.
On the negative side, sulfuric acid is a hazardous, aggressive chemical. An accidental overfeed will cause a very corrosive condition that will result in irreversible system damage. Low pH conditions will also cause deterioration of concrete basins, steel pipe and galvanized steel cooling towers. For these reasons, the use of acid is generally restricted to very high hardness and alkalinity conditions.
As mentioned previously, calcium in the makeup restricts cycles of concentration to a practical limit of 350 to 450 ppm. A makeup supply that has 100 ppm calcium hardness, for example, limits the cooling tower cycles to 3.5 to 4.5. A practical way to remove this restriction is to soften the makeup to remove the calcium and magnesium hardness. An industrial or commercial water softener removes essentially all of the hardness and iron from the makeup. This water can be used “as is” or blended with a percentage of raw water to produce a final makeup of any desired hardness. Reducing the hardness to 50 ppm by softening and blending would permit the tower in our example to operate at seven to nine cycles instead of 3.5 to 4.5.
Softening offers another advantage in that it does not remove the alkalinity nor depress the pH. The high alkalinity and pH of the cooling water tends to passivate (make less prone to corrosion) steel, copper and galvanized steel. The higher pH also serves to inhibit bacteria growth in that many organisms do not thrive at pH values above nine.
Benefits of improved maintenance Maintaining cooling towers at maximum cycles of concentration is a practical way to conserve on freshwater withdrawal rates and produce less wastewater. This has a secondary benefit in that operating a cooling tower more efficiently reduces chemical treatment requirements and saves energy.
The maintenance team plays a vital role in helping to increase profit, gain competitive advantage and protect the natural environment. Water and energy conservation are at the core of this effort. These simple upgrades in cooling tower maintenance practices will help achieve these goals now and into the future. MT
William F. Harfst, president of Harfst and Associates, Inc., has 35 years of water management experience. An independent consultant, he works with industrial, institutional, government, utility and commercial clients on projects that conserve water, minimize waste, reduce chemical consumption and save energy. His strategies focus on increasing profit and gaining competitive advantage for his clients by implementing water management programs that protect and enhance the natural environment. Telephone: (815) 477-4559; e-mail: wfh@mc.net






View Comments